|
Ribose
is a pentose (five-carbon sugar) that is a component
of the ribonucleic acid (RNA), where it alternates with
phosphate groups to form the 'back-bone' of the RNA
polymer and binds to nitrogenous bases. Ribose phosphates
are components of the nucleotide coenzymes and are utilized
by microorganisms in the synthesis of the amino acid
histidine. Its close relative, deoxyribose, is a constituent
of deoxyribonucleic acid (DNA), where it alternates
with phosphate groups to form the 'back-bone' of the
DNA polymer and binds to nitrogenous bases. The presence
of deoxyribose instead of ribose is one difference between
DNA and RNA. Ribose has one more oxygen atom in its
molecule than deoxyribose. Ribose has a five member
ring composed of four carbon atoms and one oxygen. Hydroxyl
groups are attached to three of the carbons. The other
carbon and a hydroxyl group are attached to one of the
carbon atoms adjacent to the oxygen. In deoxyribose,
the carbon furthest from the attached carbon is stripped
of the oxygen atom in what would be a hydroxyl group
in ribose. The sugar (ribose or deoxyribose) molecules
in the nucleic acid are all oriented in the same direction.
Their carbon atoms are numbered: the 5' carbon atom
is always on the side of the sugar molecule that faces
the leading end, while the 3' carbon atom always faces
the tail end. Nucleotide is the structural unit of a
nucleic acid. A nucleotide consists of either a nitrogenous
heterocyclic base (purine or pyrimidine) , a pentose
sugar (ribose or deoxyribose) and a phosphate group
attached at the 5' position on the sugar. A nucleoside
consists of only a pentose sugar linked to a purine
or pyrimidine base, without a phosphate group. Purine
bases are Adenine, Guanine and Hypoxanthine (examples
of purine nucleosides are Adenosine, 2'-Deoxyadenosine,
Guanosine, 2'-Deoxyguanosine, Inosine, 2'-Deoxyinosine).
Pyrimidine bases are Cytosine, Thymine, and Uracil (examples
of pyrimidine nucleosides are Cytidine, 2'-Deoxyguanosine,
5-Methyluridine, 2'-Deoxy-5-Methyluridine, Uridine,
2'-Deoxyuridine). The nucleoside derivatives are involved
in important functions in cellular metabolism and are
used to synthesize enzyme inhibitors, antiviral agents,
and anticancer agents.
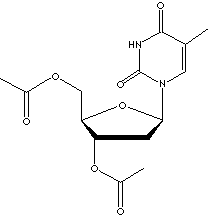
Thymine is a pyrimidine base, occurring condensed with deoxyribose to form the
nucleoside deoxythymidine in animal cells. Thymidine is significant because of its involvement in the biosynthesis of DNA
and in the preservation and transfer of genetic information. It was thought that there is no
thymine in ribonucleic acid (RNA); the thymine nucleotides contain only
deoxyribose. However, It is now known that thymine, produced from uracil by
post-transcriptional methylation, occurs as a rare base in rRNAs and tRNAs. When
N1 is linked to the C1 of ribose, thymine forms a pyrimidine
nucleoside called thymidines which are phosphorylated with from one to three
phosphoric acid groups to form the three nucleotides; thymidine
monophosphate(TMP), thymidine diphosphate(TDP), and thymidine triphosphate (TTP)
respectively. TTP is involved in the formation of adenosine triphosphate (ATP)
as a donator of phosphate groups to adenosine diphosphate (ADP).
- Thymine:
a pyrimidine base
- Thymidine: a pyrimidine nucleoside
- Thymidine
Monophosphate (TMP, Thymidylic acid): a nucleotide, the 5'-phosphate of
thymidine
- Thymidine dDphosphate (TDP): a nucleotide, the 5'-pyrophosphate of
thymidine
- Thymidine Triphosphate (TTP): a nucleotide, the 5'-triphosphate of
thymidine
- Deoxythymidine Monophosphate (dTMP), a nucleotide, the
5'-phosphate of deoxythymidine. (deoxy-, also called desoxy, is a prefix for
the designation of compounds which contain one less atom of oxygen than the
reference substance).
- Deoxythymidine
Diphosphate (dTDP), a nucleotide, the 5'-pyrophosphate of
deoxythymidine.
- Deoxythymidine Triphosphate (dTTP), a nucleotide, the 5'-triphosphate
of deoxythymidine; a precursor in the synthesis of
deoxyribonucleic acid.
Chemically modified nucleotides substituted
or attached by special chemical groups or elements are studied
and used to inactivate the normal biological operation in the living organism
and the function of important enzymes.
|
|
Purine is a heterocyclic compound featured by a fused pyrimidine and imidazole
rings composed of carbon and nitrogen atoms. The simplest one is purine itself
and the two major purines are adenine(6-Aminopurine) and
guanine(2-Amino-6-hydroxypurine) which are two bases components of nucleic acid
and the nucleotides. Purine itself is not found in nature, but as substituted
purines such as methyled, hydroxyl and amino substituted. In addition to adenine
and guanine, a group of chemical compounds called purine base include
hypoxanthine (6-oxypurine), xanthine (2,6-dioxypurine), uric acid
(2,6,8-trioxypurine), and theobromine (3,7-dimethyl xanthine). Theophylline and
caffeine are a member of methylated purine family. Purines are biologically
important in In medicine and biological research. |